.For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations.For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions.For atoms with 4 valence electrons, it can go either way.For atoms with 8 valence electrons, there is no change. Valence and Core Electrons Purpose To discover the arrangements of electrons within atoms. Instructions Name Date Period 68 Complete the table on the handout, filling in the missing atoms. Then answer the questions. How does the number of electrons change as you move from left to right across a period?
1.
A. The number of valence electrons in aluminum is
1) 1e– 2) 2e– 3) 3e–
B. The change in electrons for octet requires a
1) loss of 3e– 2) gain of 3e– 3) gain of 5e–
C. The ionic charge of aluminum is
1) 3– 2) 5– 3) 3+
D. The symbol for the aluminum ion is
1) Al3+ 2) Al3- 3) Al+
Show AnswerA. The number of valence electrons in aluminum is
3) 3e–
B. The change in electrons for octet requires a
1) loss of 3e–
C. The ionic charge of aluminum is
3) 3+
D. The symbol for the aluminum ion is
1) Al3+
2.
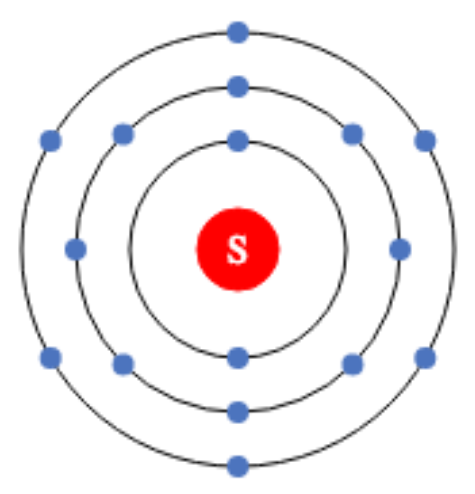
A. Number of valence electrons in sulfur is
1) 4e– 2) 6e– 3) 8e–
B. Change in electrons for octet requires a
1) loss of 2e– 2) gain of 2e– 3) gain of 4e–
C. Ionic charge of sulfur is
1) 2+ 2) 2- 3) 4-
Show AnswerA. Number of valence electrons in sulfur is
2) 6e–
B. Change in electrons for octet requires a
2) gain of 2e–
C. Ionic charge of sulfur is
2) 2-
3. Select the correct formula for each of the following ionic compounds
A. Na+ and S2-
1) NaS 2) Na2S 3) NaS2
B. Al3+ and Cl–
1) AlCl3 2) AlCl 3) Al3Cl
C. Mg2+ and N3-
1) MgN 2) Mg2N3 3) Mg3N2
Show AnswerA. Na+ and S2-
2) Na2S
check: 2Na+ + S2- = 2(1+) + 1(2-) = 0
B. Al3+ and Cl–
1) AlCl3
check: Al3+ + 3Cl– = (3+) + 3(1-) = 0
C. Mg2+ and N3-
3) Mg3N2
check: 3Mg2+ +2N3- = 3(2+) + 2(3-) = 0
4. Complete the names of the following ions:
Ba2+ Al3+ K+
_________ __________ _________
N3– O2– F–
_________ __________ _________
P3– S2– Cl–
_________ __________ __________
Show AnswerBa2+ Al3+ K+
barium aluminum potassium
N3– O2– F–
nitride oxide fluoride
P3– S2– Cl–
phosphide sulfide chloride
5. Write the names of the following compounds:
1) CaO ___________
2) KBr ___________
3) Al2O3 ___________
4) MgCl2 ___________
Show AnswerWrite the names of the following compounds:
1) CaO calcium oxide
2) KBr potassium bromide
3) Al2O3 aluminum oxide
4) MgCl2 magnesium chloride
6. Select the correct name for each.
A. Fe2S3
1) iron sulfide
2) iron(II) sulfide
3) iron (III) sulfide
B. CuO
1) copper oxide
2) copper(I) oxide
3) copper (II) oxide
Show AnswerSelect the correct name for each.
A. Fe2S3
3) iron (III) sulfide Fe3+ S2-
B. CuO
3) copper (II) oxide Cu2+ O2-
7. The correct formula for each of the following is:
A. Copper (I) nitride
1) CuN 2) CuN3 3) Cu3N
B. Lead (IV) oxide
1) PbO2 2) PbO 3) Pb2O4
Show AnswerTotal Number Of Valence Electrons In Calcium
The correct formula is
A. Copper (I) nitride
3) Cu3N 3Cu+ + N3- = 3(1+) + (3-) = 0
B. Lead (IV) oxide
1) PbO2 Pb4+ + 2O2- = (4+) +2(2-) = 0
8. Match each formula with the correct name:
A. MgS 1) magnesium sulfite
MgSO3 2) magnesium sulfate
MgSO4 3) magnesium sulfide
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 2) calcium chlorite
Ca(ClO2)2 3) calcium chloride
Show AnswerMatch each formula with the correct name:
A. MgS 3) magnesium sulfide
MgSO3 1) magnesium sulfite
MgSO4 2) magnesium sulfate
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 3) calcium chloride
Amount Of Valence Electrons In Calcium
Ca(ClO2)2 2) calcium chlorite
9. Name each of the following compounds:
A. Mg(NO3)2
B. Cu(ClO3)2
C. PbO2
D. Fe2(SO4)3
E. Ba3(PO3)2
Show AnswerName each of the following compounds:
A. Mg(NO3)2 magnesium nitrate
B. Cu(ClO3)2 copper(II) chlorate
C. PbO2 lead (IV) oxide
D. Fe2(SO4)3 iron(III) sulfate
E. Ba3(PO3)2 barium phosphite
10. Write the correct formula for each:
A. potassium bromate
B. calcium carbonate
C. sodium phosphate
D. iron(III) oxide
E. iron (II) nitrite
Show AnswerWrite the correct formula for each:
A. potassium bromate KBrO3
B. calcium carbonate CaCO3
C. sodium phosphate Na3PO4
D. iron(III) oxide Fe2O3
E. iron (II) nitrite Fe(NO2)2
11. Name the following compounds:
A. Ca3(PO4)2
B. FeBr3
C. Al2S3
D. Zn(NO2)2
E. NaHCO3
Show AnswerName the following compounds:
A. Ca3(PO4)2 Ca2+ PO43− calcium phosphate
B. FeBr3 Fe3+ Br − iron(III) bromide
C. Al2S3 Al3+ S2− aluminum sulfide
D. Zn(NO2)2 Zn2+ NO2− zinc nitrite
E. NaHCO3 Na+ HCO3− sodium hydrogen carbonate
12. Write the formulas for the following:
A. calcium nitrate
B. iron(II) hydroxide
C. aluminum carbonate
D. copper(II) bromide
E. lithium phosphate
Show AnswerWrite the formulas for the following:
A. calcium nitrate Ca2+, NO3− Ca(NO3)2
B. iron(II) hydroxide Fe2+, OH− Fe(OH)2
C. aluminum carbonate Al3+, CO32− Al2(CO3) 3
D. copper(II) bromide Cu2+, Br− CuBr2
E. lithium phosphate Li+, PO43− Li3PO4
13. Match each formula with the correct name:
A. MgS 1) magnesium sulfite
MgSO3 2) magnesium sulfate
MgSO4 3) magnesium sulfide
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 2) calcium chlorite
Ca(ClO2)2 3) calcium chloride
Show AnswerMatch each formula with the correct name:
A. MgS 3) magnesium sulfide
MgSO3 1) magnesium sulfite
MgSO4 2) magnesium sulfate
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 3) calcium chloride
Ca(ClO2)2 2) calcium chlorite
14. Name each of the following compounds:
A. Mg(NO3)2
B. Cu(ClO3)2
C. PbO2
D. Fe2(SO4)3
E. Ba3(PO3)2
Show AnswerName each of the following compounds:
A. Mg(NO3)2 magnesium nitrate
B. Cu(ClO3)2 copper(II) chlorate
C. PbO2 lead (IV) oxide
D. Fe2(SO4)3 iron(III) sulfate
E. Ba3(PO3)2 barium phosphite
15. Select the correct formula for each:
A. aluminum nitrate
1) AlNO3 2) Al(NO)3 3) Al(NO3)3
B. copper(II) nitrate
1) CuNO3 2) Cu(NO3)2 3) Cu2(NO3)
C. iron (III) hydroxide
1) FeOH 2) FeOH3 3) Fe(OH)3
D. tin(IV) hydroxide
1) Sn(OH)4 2) Sn(OH)2 3) Sn4(OH)
Show AnswerSelect the correct formula for each:
A. aluminum nitrate
3) Al(NO3)3
B. copper(II) nitrate
2) Cu(NO3)2
Number Of Valence Electrons In Calcium Atom
C. iron(III) hydroxide
3) Fe(OH)3
D. tin(IV) hydroxide
1) Sn(OH)4
16. Write the correct formula for each:
A. potassium bromate
B. calcium carbonate
C. sodium phosphate
D. iron(III) oxide
E. iron (II) nitrite
Show AnswerWrite the correct formula for each:
A. potassium bromate KBrO3
B. calcium carbonate CaCO3
C. sodium phosphate Na3PO4
D. iron(III) oxide Fe2O3
E. iron (II) nitrite Fe(NO2)2
17. Name the following compounds:
A. Ca3(PO4)2
B. FeBr3
C. Al2S3
D. Zn(NO2)2
E. NaHCO3
Show AnswerName the following compounds:
A. Ca3(PO4)2 Ca2+ PO43− calcium phosphate
B. FeBr3 Fe3+ Br − iron(III) bromide
C. Al2S3 Al3+ S2− aluminum sulfide
D. Zn(NO2)2 Zn2+ NO2− zinc nitrite
E. NaHCO3 Na+ HCO3− sodium hydrogen
18. Write the formulas for the following:
A. calcium nitrate
B. iron(II) hydroxide
C. aluminum carbonate
D. copper(II) bromide
E. lithium phosphate
Show AnswerWrite the formulas for the following:
A. calcium nitrate Ca2+, NO3− Ca(NO3)2
B. iron(II) hydroxide Fe2+, OH− Fe(OH)2
C. aluminum carbonate Al3+, CO32− Al2(CO3) 3
D. copper(II) bromide Cu2+, Br− CuBr2
E. lithium phosphate Li+, PO43− Li3PO4
1.
A. The number of valence electrons in aluminum is
1) 1e– 2) 2e– 3) 3e–
B. The change in electrons for octet requires a
1) loss of 3e– 2) gain of 3e– 3) gain of 5e–
C. The ionic charge of aluminum is
1) 3– 2) 5– 3) 3+
D. The symbol for the aluminum ion is
1) Al3+ 2) Al3- 3) Al+
Show AnswerA. The number of valence electrons in aluminum is
3) 3e–
B. The change in electrons for octet requires a
1) loss of 3e–
C. The ionic charge of aluminum is
3) 3+
D. The symbol for the aluminum ion is
1) Al3+
2.
A. Number of valence electrons in sulfur is
1) 4e– 2) 6e– 3) 8e–
B. Change in electrons for octet requires a
1) loss of 2e– 2) gain of 2e– 3) gain of 4e–
C. Ionic charge of sulfur is
1) 2+ 2) 2- 3) 4-
Show AnswerA. Number of valence electrons in sulfur is
2) 6e–
B. Change in electrons for octet requires a
2) gain of 2e–
C. Ionic charge of sulfur is
2) 2-
3. Select the correct formula for each of the following ionic compounds
A. Na+ and S2-
1) NaS 2) Na2S 3) NaS2
B. Al3+ and Cl–
1) AlCl3 2) AlCl 3) Al3Cl
C. Mg2+ and N3-
1) MgN 2) Mg2N3 3) Mg3N2
Show AnswerA. Na+ and S2-
2) Na2S
check: 2Na+ + S2- = 2(1+) + 1(2-) = 0
B. Al3+ and Cl–
1) AlCl3
check: Al3+ + 3Cl– = (3+) + 3(1-) = 0
C. Mg2+ and N3-
3) Mg3N2
check: 3Mg2+ +2N3- = 3(2+) + 2(3-) = 0
4. Complete the names of the following ions:
Ba2+ Al3+ K+
_________ __________ _________
N3– O2– F–
_________ __________ _________
P3– S2– Cl–
_________ __________ __________
Show AnswerBa2+ Al3+ K+
barium aluminum potassium
N3– O2– F–
nitride oxide fluoride
P3– S2– Cl–
phosphide sulfide chloride
5. Write the names of the following compounds:
1) CaO ___________
2) KBr ___________
3) Al2O3 ___________
4) MgCl2 ___________
Show AnswerWrite the names of the following compounds:
1) CaO calcium oxide
2) KBr potassium bromide
3) Al2O3 aluminum oxide
4) MgCl2 magnesium chloride
6. Select the correct name for each.
A. Fe2S3
1) iron sulfide
2) iron(II) sulfide
3) iron (III) sulfide
B. CuO
1) copper oxide
2) copper(I) oxide
3) copper (II) oxide
Show AnswerSelect the correct name for each.
A. Fe2S3
3) iron (III) sulfide Fe3+ S2-
B. CuO
3) copper (II) oxide Cu2+ O2-
7. The correct formula for each of the following is:
A. Copper (I) nitride
1) CuN 2) CuN3 3) Cu3N
B. Lead (IV) oxide
1) PbO2 2) PbO 3) Pb2O4
Show AnswerThe correct formula is
A. Copper (I) nitride
3) Cu3N 3Cu+ + N3- = 3(1+) + (3-) = 0
B. Lead (IV) oxide
1) PbO2 Pb4+ + 2O2- = (4+) +2(2-) = 0
8. Match each formula with the correct name:
A. MgS 1) magnesium sulfite
MgSO3 2) magnesium sulfate
MgSO4 3) magnesium sulfide
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 2) calcium chlorite
Ca(ClO2)2 3) calcium chloride
Show AnswerMatch each formula with the correct name:
A. MgS 3) magnesium sulfide
MgSO3 1) magnesium sulfite
MgSO4 2) magnesium sulfate
B. Ca(ClO3)2 1) calcium chlorate

CaCl2 3) calcium chloride
Ca(ClO2)2 2) calcium chlorite
9. Name each of the following compounds:
A. Mg(NO3)2
B. Cu(ClO3)2
C. PbO2
D. Fe2(SO4)3
E. Ba3(PO3)2
Show AnswerName each of the following compounds:
A. Mg(NO3)2 magnesium nitrate
B. Cu(ClO3)2 copper(II) chlorate
C. PbO2 lead (IV) oxide
D. Fe2(SO4)3 iron(III) sulfate
E. Ba3(PO3)2 barium phosphite
10. Write the correct formula for each:
A. potassium bromate
B. calcium carbonate
C. sodium phosphate
D. iron(III) oxide
E. iron (II) nitrite
Show AnswerWrite the correct formula for each:
A. potassium bromate KBrO3
B. calcium carbonate CaCO3
C. sodium phosphate Na3PO4
D. iron(III) oxide Fe2O3
E. iron (II) nitrite Fe(NO2)2
11. Name the following compounds:
A. Ca3(PO4)2
B. FeBr3
C. Al2S3
D. Zn(NO2)2
E. NaHCO3
Show AnswerName the following compounds:
A. Ca3(PO4)2 Ca2+ PO43− calcium phosphate
B. FeBr3 Fe3+ Br − iron(III) bromide
C. Al2S3 Al3+ S2− aluminum sulfide
D. Zn(NO2)2 Zn2+ NO2− zinc nitrite
E. NaHCO3 Na+ HCO3− sodium hydrogen carbonate
12. Write the formulas for the following:
A. calcium nitrate
B. iron(II) hydroxide
C. aluminum carbonate
D. copper(II) bromide
E. lithium phosphate
Show AnswerWrite the formulas for the following:
A. calcium nitrate Ca2+, NO3− Ca(NO3)2
B. iron(II) hydroxide Fe2+, OH− Fe(OH)2
C. aluminum carbonate Al3+, CO32− Al2(CO3) 3
D. copper(II) bromide Cu2+, Br− CuBr2
E. lithium phosphate Li+, PO43− Li3PO4
13. Match each formula with the correct name:
A. MgS 1) magnesium sulfite
MgSO3 2) magnesium sulfate
MgSO4 3) magnesium sulfide
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 2) calcium chlorite
Ca(ClO2)2 3) calcium chloride
Show AnswerMatch each formula with the correct name:
A. MgS 3) magnesium sulfide
MgSO3 1) magnesium sulfite
MgSO4 2) magnesium sulfate
B. Ca(ClO3)2 1) calcium chlorate
CaCl2 3) calcium chloride
Ca(ClO2)2 2) calcium chlorite
14. Name each of the following compounds:
A. Mg(NO3)2
B. Cu(ClO3)2
C. PbO2
D. Fe2(SO4)3
E. Ba3(PO3)2
 Show Answer
Show AnswerName each of the following compounds:
A. Mg(NO3)2 magnesium nitrate
B. Cu(ClO3)2 copper(II) chlorate
C. PbO2 lead (IV) oxide
D. Fe2(SO4)3 iron(III) sulfate
E. Ba3(PO3)2 barium phosphite
15. Select the correct formula for each:
A. aluminum nitrate
1) AlNO3 2) Al(NO)3 3) Al(NO3)3
B. copper(II) nitrate
1) CuNO3 2) Cu(NO3)2 3) Cu2(NO3)
C. iron (III) hydroxide
1) FeOH 2) FeOH3 3) Fe(OH)3
D. tin(IV) hydroxide
1) Sn(OH)4 2) Sn(OH)2 3) Sn4(OH)
Valence Electrons Calculator
Show AnswerSelect the correct formula for each:
A. aluminum nitrate
3) Al(NO3)3
B. copper(II) nitrate
2) Cu(NO3)2
C. iron(III) hydroxide
3) Fe(OH)3
D. tin(IV) hydroxide
1) Sn(OH)4
16. Write the correct formula for each:

A. potassium bromate
B. calcium carbonate
C. sodium phosphate
D. iron(III) oxide
E. iron (II) nitrite
Show AnswerWrite the correct formula for each:
A. potassium bromate KBrO3
B. calcium carbonate CaCO3
C. sodium phosphate Na3PO4
D. iron(III) oxide Fe2O3
E. iron (II) nitrite Fe(NO2)2
17. Name the following compounds:
A. Ca3(PO4)2
B. FeBr3
C. Al2S3
D. Zn(NO2)2
E. NaHCO3
Show AnswerThe Number Of Valence Electrons In Calcium
Name the following compounds:
A. Ca3(PO4)2 Ca2+ PO43− calcium phosphate
B. FeBr3 Fe3+ Br − iron(III) bromide
C. Al2S3 Al3+ S2− aluminum sulfide
D. Zn(NO2)2 Zn2+ NO2− zinc nitrite
E. NaHCO3 Na+ HCO3− sodium hydrogen
The Number Of Valence Electrons In Calcium
18. Write the formulas for the following:
A. calcium nitrate
B. iron(II) hydroxide
C. aluminum carbonate
D. copper(II) bromide
E. lithium phosphate
Show AnswerWrite the formulas for the following:
A. calcium nitrate Ca2+, NO3− Ca(NO3)2
B. iron(II) hydroxide Fe2+, OH− Fe(OH)2
C. aluminum carbonate Al3+, CO32− Al2(CO3) 3
D. copper(II) bromide Cu2+, Br− CuBr2
E. lithium phosphate Li+, PO43− Li3PO4
